
Mon - Sat 9:00 - 17:30

As industrial safety professionals, we are all familiar with the term flash point. By definition, Flash Point is the lowest temperature at which a liquid gives off enough vapors to form an ignitable mixture in air near the surface of the liquid under specified test conditions. It is a critical property used to classify flammable and combustible liquids, referenced in numerous fire safety codes and standards.
But while we often quote the flash point values from material safety data sheets (MSDS) or standards, an important question remains: how is flash point determined, and under what conditions?
The measurement of flash point is standardized and carried out using defined apparatus. The two commonly used methods are:
Closed Cup Method (e.g., Pensky–Martens, Abel, Tagliabue)
In this method, the test sample is enclosed, and the vapors are in equilibrium with the liquid before ignition is attempted.
Generally gives lower values than open cup.
Standards: ASTM D93 (Pensky–Martens), ASTM D56 (Tag Closed Cup).
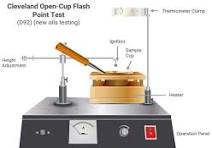
Open Cup Method (e.g., Cleveland Open Cup)
The liquid is exposed to air during heating, and ignition is tested at intervals.
Tends to give higher flash points since vapors can disperse.
Standard: ASTM D92.
Both methods have their defined scope, and selection depends on the type of liquid and regulatory requirements.
It is important to note that flash point values are determined under standard laboratory conditions:
Temperature: 25 °C (298 K)
Pressure: 1 atmosphere (101.3 kPa or 760 mmHg)
This ensures reproducibility and comparability. But in real-world applications, conditions may not always remain the same.
Flash point is directly linked to vapor pressure. Since ignition occurs when vapor concentration reaches the lower flammable limit (LFL), any change in ambient pressure affects vapor formation.
At Higher Pressure (e.g., pressurized storage or low-lying areas):
Vaporization is suppressed. Hence, the liquid requires a slightly higher temperature to produce enough vapor, raising the flash point.
At Lower Pressure (e.g., high-altitude / hilly regions):
Liquids vaporize more easily due to reduced atmospheric pressure. Therefore, the flash point tends to decrease, meaning a liquid may ignite at lower temperatures than expected.
Consider Gasoline (Petrol):
Flash Point: approximately –40 °C (closed cup, ASTM D56, NFPA 30 reference) at sea level (101.3 kPa).
Classification: NFPA 30 classifies gasoline as a Class I flammable liquid (flash point < 37.8 °C).
If the same fuel is handled in a high-altitude refinery (say at ~2,000 m elevation, where atmospheric pressure drops to ~80 kPa), its flash point would be observed at a slightly lower value because vapors form more readily.
This implies that flash point values reported on MSDS may not directly reflect behavior in actual site conditions, especially for facilities located in high-altitude or pressurized environments.
When selecting a liquid for process use, storage, or transportation, engineers and safety professionals must consider not just the reported flash point, but also:
Altitude of the facility (sea level vs. high-altitude plant).
Operating pressure conditions in tanks or closed systems.
Environmental temperature variations that can amplify risks.
Ignoring these can lead to underestimation of hazards, flawed area classification, and ineffective design of fire protection systems.
ASTM D92: Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester.
ASTM D93: Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester.
ASTM D56: Standard Test Method for Flash Point by Tag Closed Cup Tester.
NFPA 30 (2021): Flammable and Combustible Liquids Code.
ISO 2719: Determination of flash point – Pensky–Martens closed cup method.
ILO International Chemical Safety Cards – Flash point & vapor pressure behavior notes.
✅ Key Takeaway: Flash point is not an absolute number—it is pressure and temperature dependent. As safety professionals, we must account for altitude, storage pressure, and real-world conditions when interpreting flash points and designing fire & explosion protection measures.